Human factors research
Engineer safety. Reduce risk.
Align with how people really use your product.
We help you deliver safer, more intuitive medical products by embedding human factors (HF) research throughout your development process. From concept to FDA submission, we design programs that identify risk, inform design, and validate usability in real-world conditions.
Beyond compliance: Turn human factors into a competitive edge
We don’t treat HF like a checkbox. We integrate it as a strategic advantage to help you:
What we do
We’ve applied human factors engineering (HFE) to medical device development longer than the FDA has regulated it! Our research adheres to global standards like IEC 62366-1, TR 62366-2, FDA HFE/UE guidance, and AAMI HE75.
Our HF research supports high-stakes decisions across your product lifecycle:

From the field
In high-pressure simulations, we saw how crash cart packaging can make or break clinical response time. By observing real-world workflows and testing design concepts under urgent conditions, we uncovered critical barriers and shaped packaging that helps teams move faster, safer, and with greater confidence.
Simulated-use testing
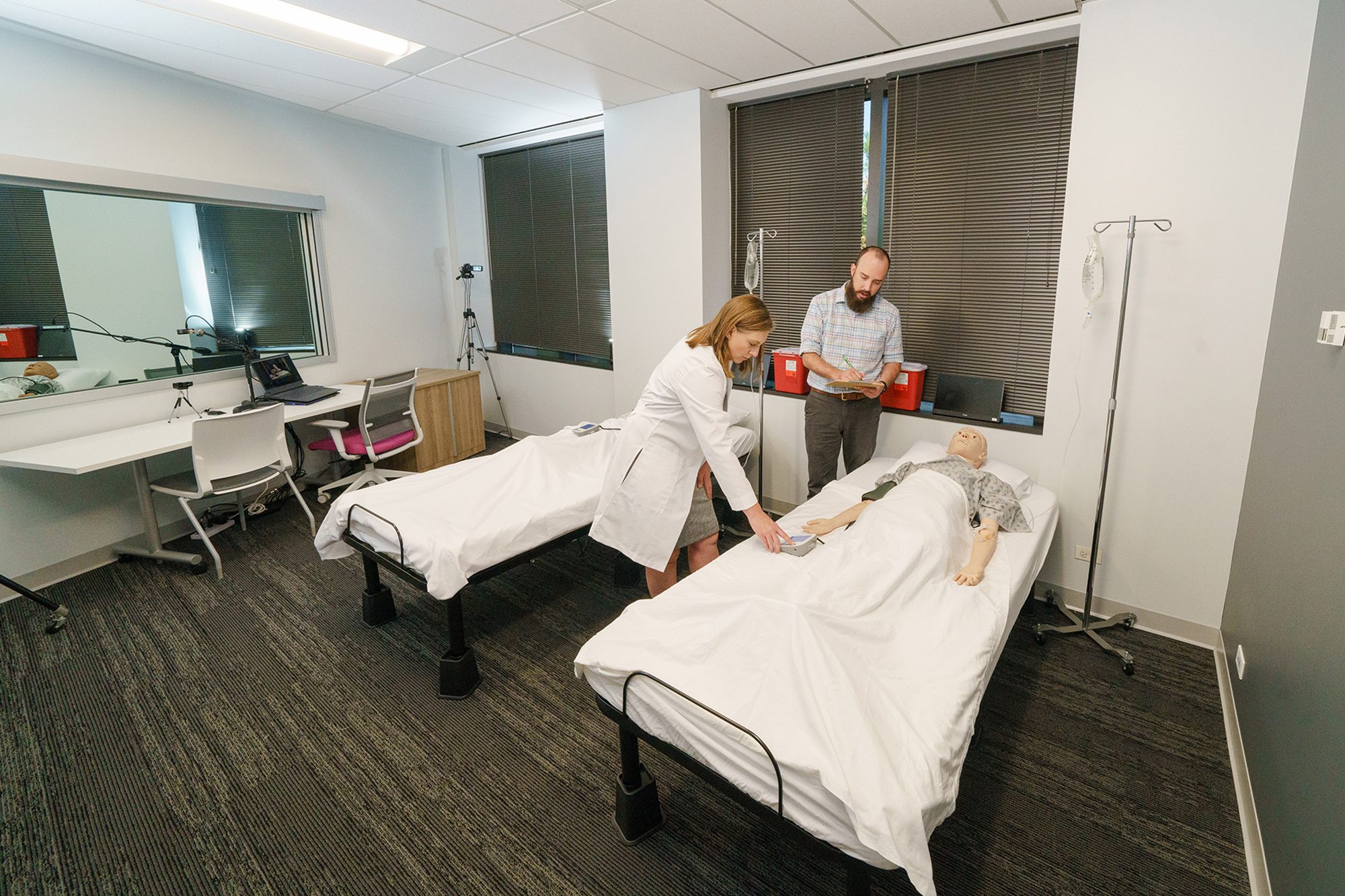
Replicate real-world scenarios to evaluate product safety and usability. From in-house labs with screen mirroring and one-way mirrors to fully equipped OR/ICU mockups and live clinical observation, we match the fidelity to your product, users, and goals.
Formative research
Identify and address use-related risks early in development. Our studies uncover pain points, inform design decisions, and de-risk your product path.

From the field
In a simulated clinical setting, we observed oncology providers using a new on-body delivery system. By pinpointing where instructions caused confusion and identifying steps at risk for error, we delivered clear direction to refine the IFU and protocol, setting the stage for safer use, smoother validation, and a stronger regulatory submission.

From the field
To help bring a neonatal breathing circuit safely to market, we recreated the real-world clinical environment in our Chicago lab, complete with a ventilator, incubator, and care team simulation. Through summative testing with experienced healthcare providers, we identified and addressed potential use errors, delivering the validation data our client needed for regulatory approval.
Summative validation
Support submissions with HF validation studies aligned to regulatory standards. We design and run rigorous tests to show your product is safe, effective for use, and ready for market.
Labeling & IFU testing
Evaluate how users interpret packaging, instructions for use (IFU), and warnings, ensuring clarity and compliance. Our team specializes in IFU and packaging design, adhering to regulatory guidance and user-centered design best practices.
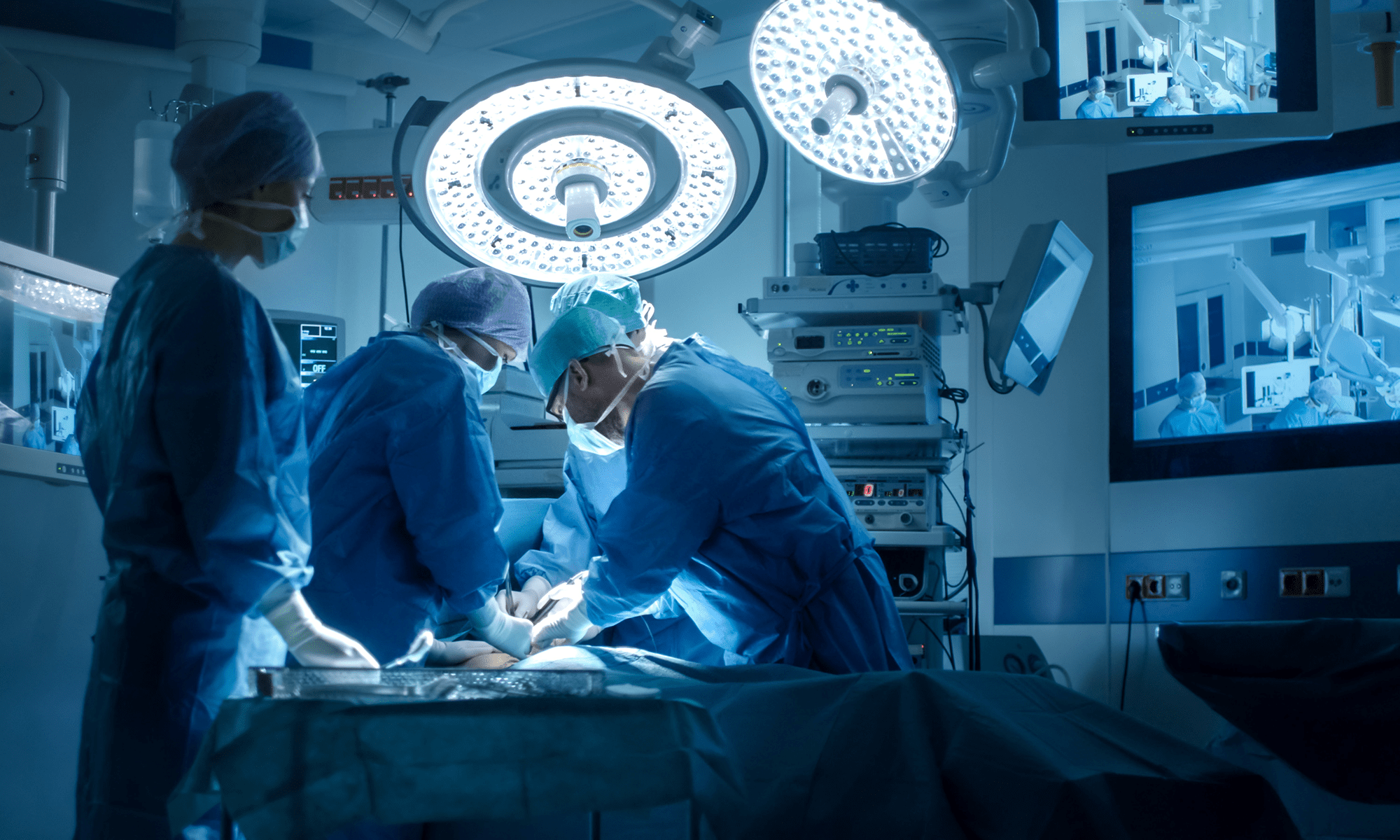
From the field
In a high-fidelity hospital simulation, we watched surgical teams put a next-gen mesh implant through its paces. By observing real-time interactions with the updated procedure and packaging, we uncovered critical usability gaps and points of confusion. Our insights led to redesigned instructions and labeling that improved clarity, reduced risk, and helped ensure safer, more consistent implantations across clinical teams.

From the field
Partnering closely with a medical device manufacturer, we embedded an HF engineer into the product team to drive usability and regulatory readiness from the inside out. From crafting submission-ready HFE documentation to running a summative validation study with healthcare providers, we ensured the device’s safe, effective use was clearly demonstrated. The result? A confident FDA submission backed by robust usability evidence.
Augment your team
Need embedded HF experts? We integrate seamlessly with your product and regulatory teams to provide strategic support and documentation from concept through commercialization.
Why us
We specialize in the messy, real-world environments where your product will live.

Regulatory expertise, global reach
We’ve supported HF submissions in around the world. Our ISO 9001:2015–certified system ensures quality and audit readiness no matter what location is required.

Built for high-risk use cases
We thrive in regulated, multi-user, high-complexity environments, from at-home autoinjectors to hospital-based surgical systems.

Designed for action
You get more than a report. We deliver task flows, journey maps, risk mitigation plans, and submission-ready documentation.
What we deliver
Whether you’re refining an IFU, validating a digital therapeutic, or preparing for submission, we bring the clarity, rigor, and experience to help you succeed in the real world.
Blogs
Human factors research
These blogs share lessons from the field and offer strategies to reduce risk and improve usability. We discuss how human factors research supports regulatory success, from formative testing to validation.











