Human factors research
Applying human factors best practices to product development is much more than a validation study. Yes, we have the expertise to plan, manage, and execute your human factors validation study… but creating safer and more efficient, effective, and engaging experiences for your customers starts much earlier than that and so do we.

17![]() 20
20
pharmaceutical companies have trusted us with their UX and human factors research
35+
senior team members
with advanced degrees or equivalent experience in human factors
25+
FDA or CE mark (EU) submissions supported each year
100+
exploratory, formative, or summative HF research engagements each year
Our human factors research informs the development and commercialization of safe and effective medical devices.
Human factors includes both cognitive usability and physical ergonomics aspects of human-product interaction. Cognitive usability refers to memory, attention, perception, decision making, detection, situation awareness, automaticity, compatibility, etc. Physical ergonomics refers to coordination, strength, posture, motor schema, dexterity, force, reach, etc. We work to identify where these aspects break down when your users use your product and how to resolve these issues.
Testing early and often allows you to guide your design using input, making for fewer surprises at the end. In most cases, it is important to test with representative users, even if the system is not fully developed.
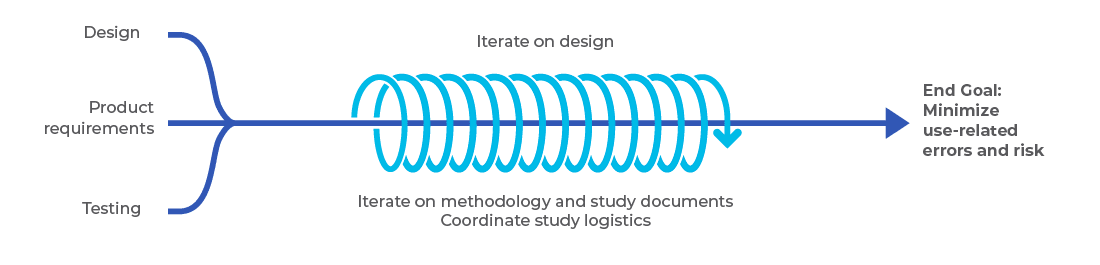
What do we measure?
Understand
- End user needs
- Context of use
- Human factors plan inputs
Plan
- The right questions to ask
- The best methods to answer your questions
- The key milestones to achieve success
Execute
- Field research to learn
- Formative user research to inform the design
- Human factors validation of safe and effective use
We often assume users will behave the way we expect them to behave. Unfortunately for us, humans are unpredictable … attempts to model behavior typically predict fewer than 10% of actions, and what people say they do is often vastly different from what they actually do. Therefore, we need to see behavior. Putting a product in front of a human and giving them a chance to use it – to behave – yields insights that cannot be predicted or hypothesized without testing. Lesson: User testing and iteration matters.
While exploratory and formative human factors research can be conducted with an eye towards achieving a competitive advantage and imperative in ensuring you have a product that is ready for validation or limiting surprises when you get to validation, a summative human factors study must be conducted to fulfill the FDA regulatory requirement of demonstrating that a medical or drug delivery device can be used safely and effectively before going to market.
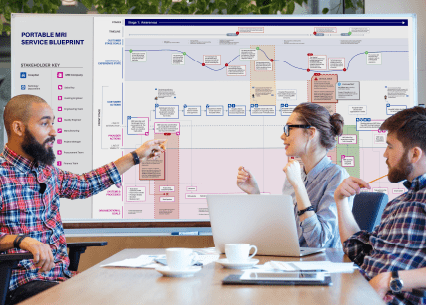
Unlike clinical trials, our research is typically conducted in a lab setting that reflects real-world use through simulated scenarios. We observe users as they complete critical tasks with the device(s) and determine (for example) the root cause of any observed use-related issues. We also provide design recommendations to overcome those areas where risk can be mitigated, as well as help get your product to the point where data show the residual risk is as low as reasonably practicable (ALARP). Human factors research is completed in a relatively short amount of time, with data collection typically requiring between 1-2 weeks, compared to months or years for a clinical trial.
Understand the unmet needs of your customers to design the right product or service.
Get feedback early and often to course correct while it’s still easy to do so.
Assess current state to focus future development efforts or to document achievement of objectives.
How do we measure?
If you’re unfamiliar with typical human factors research methods, find examples below of what each method is, what it measures, when to use it, and outcomes.
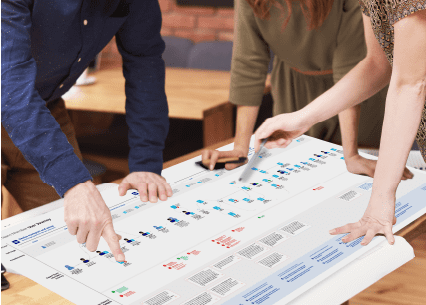
Read more about our simulated research labs where we conduct in-lab and remote testing encompassing all stages of the product development lifecycle.
Ethnography
Explore user behavior through naturalistic observation to better understand operational context of use and uncover workflows. Ethnographic research methods gather in-situ observations to develop a deeper understanding of user behaviors and processes as they interact with a product, system, or service.
When is it used?
Ethnographic methods are used in the early stages of product development.
Types of deliverables:
Detailed research findings with actionable recommendations of where to focus future development efforts.
Research questions answered:
- Rich, in-depth understanding of context of use, perceptions, motivations and thought processes driving user behaviors and workflows.
- Identification of inefficiencies in processes, tools, and designs to pinpoint areas of improvement.
- Understanding of unmet needs to inform the design of the right product or service.
- Identification of unexpected issues that may not be encountered in recall-based methods.
- Visibility into in-the-moment behaviors that can be forgotten or overlooked by users, such as habitual or obscured behaviors, in recall-based methods.
- Behavioral observation offering insights about the experience of the product, removing opinion and reporting bias.
Contextual inquiry
Take a deep dive into use scenarios to capture rich, in-the-moment data through direct observation of users’ behaviors in their natural environment. Contextual inquiry is an ethnographic method employing direct observation coupled with in-depth interviews to gain a holistic understanding of user practices and behaviors.
When is it used?
Contextual inquiry is conducted in the early stages of product development.
Research questions answered:
- Rich, in-depth understanding of users’ context of use and environment.
- Identification of regularly employed tasks, workflows and tools to help define requirements for a product as well as identify inefficiencies in those areas.
- Understanding of perceptions, motivations and thought processes driving user behaviors and workflows to identify what is important and critical to users.
- Visibility into in-the-moment behaviors that can be forgotten or overlooked by users, such as habitual or obscured behaviors, in recall-based methods.
- Identification of unexpected issues that may not be encountered in recall-based methods and unmet needs to inform the design of the right product or service.
Diary studies
An ethnographic method, diary studies are used to document the evolution of an experience over an extended period of time to identify opportunities for enhancement. Diary studies unveil the attitudes of users as they engage with products and services and log their activities as they occur.
When is it used?
Diary studies are typically conducted in the early stages of product development.
Research questions answered:
- Rich, in-depth understanding of users’ context of use and environment over a period of time.
- Identification of regularly employed tasks, workflows, and tools to help define requirements for a product as well as identify inefficiencies in those areas.
- Understanding of intricacies of tasks and workflows that are unveiled over time and with continued use.
- Understanding of perceptions, motivations and thought processes driving user behaviors and workflows to identify what is important and critical to users.
- Visibility into in-the-moment behaviors that can be forgotten or overlooked by users, such as habitual or obscured behaviors, in recall-based methods.
- Identification of unexpected issues that may not be encountered in recall-based methods and unmet needs to inform the design of the right product or service.
Time & motion studies
Assess the effects of system design on use-related processes by quantifying the impact of process improvements. Time and motion metrics are collected to examine how tasks are done, track the time it takes to complete sub-tasks within a larger task and inform the balance of sub-tasks.
When is it used?
Time and motion studies are conducted in the early stages of product development.
Research questions answered:
- Definition of a process that allows time-efficient task completion while honoring the needs of users performing the tasks.
- Identification of inefficiencies and opportunities to create a more effective process.
- Development of predictions of how long tasks should take to perform and balancing of sub-tasks.
- Tracking of the time it takes to complete each sub-task in a larger task to help with predictions of how long the overall task should take to perform. In addition, it can be used to balance sub-tasks—if you have one sub-task that takes five minutes and another sub-task that takes ten minutes, you would need two people performing the ten-minute sub-task to keep up with one person working on the five-minute sub-task.
Formative usability testing
Progress product design rapidly with insights gleaned from small-scale iterative usability testing and prepare for future validation. Formative usability testing identifies what works well and what does not in an interface or product and why issues occur.
When is it used?
Formative usability testing is conducted iteratively throughout the early stages of product development on stimuli from sketches to prototypes, and the instructions for use (IFU) and packaging as part of the product system (IFU design, iterative testing of IFUs, packaging, differentiation studies, etc.).
Research questions answered:
- Discovery of usability issues and understanding of why they occur to inform design decisions.
- Early and continuous feedback to allow development teams to improve initial designs and create well-crafted experiences.
- Behavioral observation offering insights about the experience of the product, removing opinion and reporting bias.
- Reduction of the risk of product failure in the market.
Heuristic evaluations
Focus future research and create a foundation for comparative analyses with an assessment of the extent to which a design complies with or violates heuristics. Heuristic evaluations measure a design against predetermined, recognized usability principles.
When is it used?
Heuristic evaluations are conducted in the early to middle stages of product development.
Research questions answered::
- Identification of usability issues and indication of heuristic violations with actionable solution recommendations.
- Development of a usability baseline for comparative analyses.
Benchmarking
Understand how the usability of your product compares to predicate or similar products by conducting benchmarking studies. Benchmarking measures a product’s user experience in comparison to a preceding version of the product, competitors, industry standards, or determined goals.
When is it used?
Benchmarking is performed at the end of the product development lifecycle on a fully developed design.
Research questions answered:
- Health assessment of the core product experience.
- Establishment of the product’s baseline usability by which to measure future iterations.
- Measurement of improvements and documentation of objectives and key results (OKR) to track changes over time.
- Insight into a product’s standing in the market to assess competitive advantages, disadvantages and opportunities.
- Prioritization of improvements to inform the product roadmap.
Human factors validation testing
Validate that your medical device can be used safely and effectively by its intended users, for its intended uses in its intended use environments. Human factors validation testing focuses on evaluating the critical tasks as defined by a product’s use-related risk analysis. A human factors validation test is a specific type of summative usability test.
When is it used?
Human factors validation testing is conducted at the end of product development on a fully functioning product.
Research questions answered:
- Assessment if a device is safe and effective for use with any remaining risk as residual.
Longitudinal Studies
Understand how behaviors and use are shaped over time. Longitudinal studies can start at many different points, from the initial unboxing of a product to evaluating a design concept, and understand how users change, adapt or improve use over time. These studies can be iterative in nature and typically involve interviewing the same users over weeks or months.
When is it used?
Longitudinal studies are used throughout the entire product or design lifecycle.
Research questions answered:
- Rich, in-depth understanding of users’ context of use and environment over a period of time.
- Changes in user attitudes and/or understanding with experience and exposure
- A glimpse into understanding how a novice becomes an expert

What is our output?
Our human factors reporting reflects your needs. We frequently work with manufacturers in the heavily regulated healthcare space with strict guidance on research reports. We also produce data-driven design recommendations in a variety of fidelities and formats from task flows and journey maps to 8-section HFE/UE reports .
For medical device manufacturers, industry standards and regulatory guidance demand validation of safe and effective use through application of human factors engineering and usability engineering (HFE/UE). We have been applying HFE/UE to medical device development longer than the FDA has been regulating it! Our research is conducted according to the guidelines found in IEC 62366-1, TR 62366-2, FDA Guidance on applying HFE/UE to medical device development, AAMI HE75, and other standards wherever appropriate.
Related content
Read our latest news & insights
Case studies
Beyond the data dump: Crafting research reports that drive real change
Effective research reports do more than present data. Learn how to structure reports that connect insights to business impact and inspire next steps.
Storytelling for strategic UX reporting: Drive action and engage stakeholders
Learn a three-step process to transform your research reports into compelling stories that communicate insights with clarity and impact.
Case studies