Medical device research
Trusted research partners for every stage of medical device development
Whether you’re designing a wearable injector or a robotic surgical system, we help you build safer, more effective experiences for patients and healthcare professionals. Our research spans discovery, design, validation, and post-market evaluation, delivering the insights and evidence you need to make confident product decisions and accelerate regulatory approvals.


Where human-centered design meets regulatory depth
You don’t need another vendor. You need a partner who understands the stakes.
We combine regulatory fluency with usability research to de-risk your product, support global submissions, and bring innovation to life. We’ve helped hundreds of teams move from concept to clearance with data that stands up to FDA, EMA, and NMPA scrutiny.
What we do
We tailor our approach to your product class, user profiles, and regulatory path:
Where we work
We test where your product is used. From ORs and infusion suites to in-home simulations and high-fidelity labs, our research environments are designed to reflect real-world conditions.
We run studies in:
- Hospitals and long-term care facilities
- Global in-home and outpatient settings
- Simulated-use labs
- Specialized simulated surgical suites
- Remote and hybrid contexts
From the field
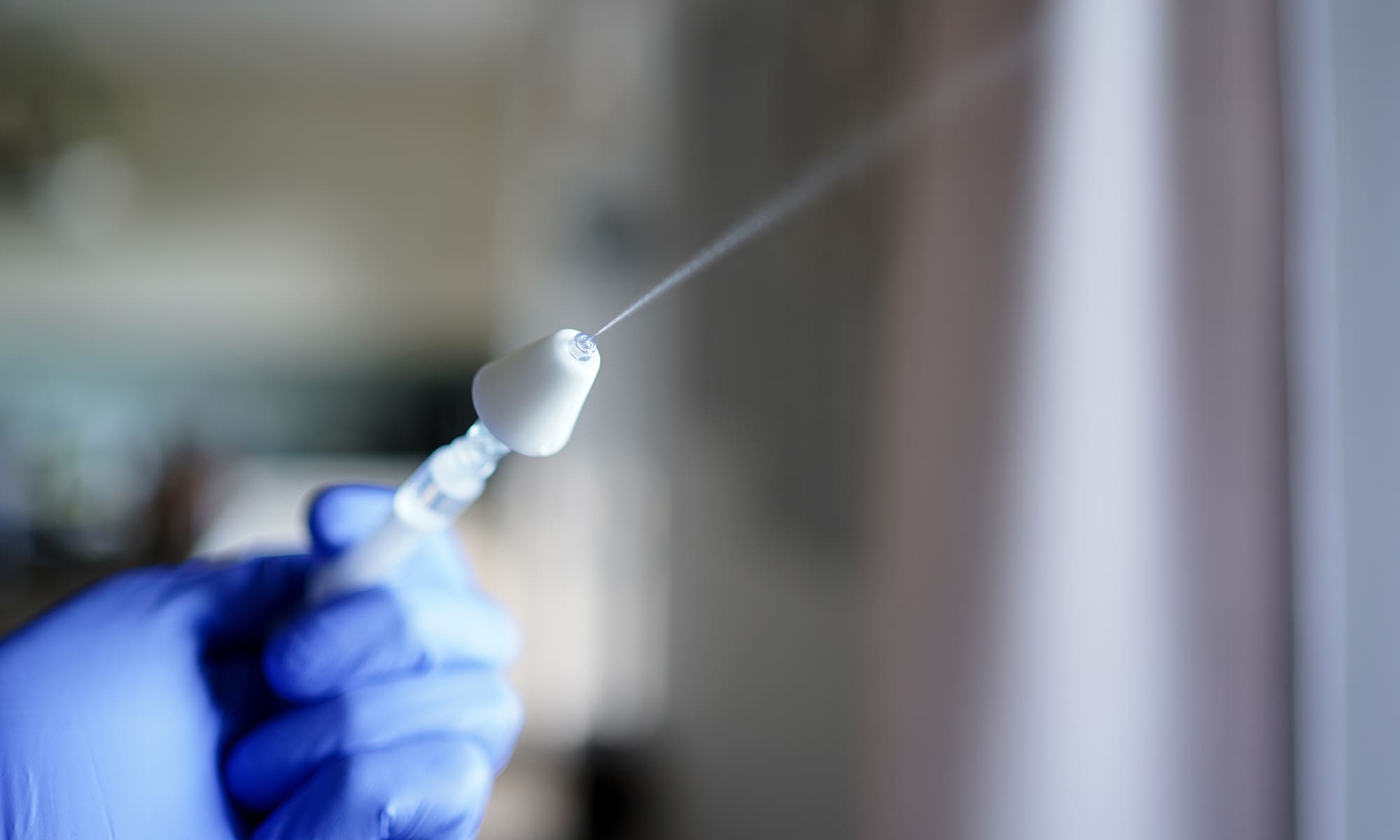
Validation in the OR
In simulated high-stress emergencies, we observed laypeople and healthcare providers use an emergency nasal MAD kit to deliver life-saving medication. Across all groups, the device met safety and usability thresholds with no critical errors, giving the manufacturer confidence for FDA submission.

Wearable infusion usability
When patients mapped their ideal journey with a wearable injection system, we saw exactly where guidance built confidence, and where gaps created hesitation. Tracking their experience from unboxing to disposal, we pinpointed key moments to refine onboarding, app integration, and design so the system fit naturally into their lives.

Tube-based oral delivery
We tested patient and caregiver comprehension of an oral medication delivery kit used with G/NG tubes. Findings improved packaging, training, and device labeling before submission.
Why us
Whether you’re designing for at-home self-administration, refining training, or navigating regulatory submission, we’re here to help. Partner with a team that brings human-centered thinking and real-world expertise to every stage of device development.