IFU Scorecard
Avoiding costly redesigns or revalidation
Labeling isn’t just packaging; it’s patient safety. Our proprietary IFU Scorecard evaluates your instructions for use (IFUs), labels, and related materials against over 600 global regulatory requirements and usability best practices. The result: a smoother FDA review, stronger design decisions, and safer user experiences.

A smarter way to evaluate labeling
Labeling is one of the most common reasons FDA reviewers request additional information during HF submissions. Our IFU Scorecard helps you catch issues before they become roadblocks.
We offer a fast, objective, and systematic assessment of your labeling materials, whether you’re at early concept or pre-validation. The scorecard helps you:
What we evaluate
Our scorecard assesses instructional materials across two dimensions. Each report includes two composite scores—“letter” and “spirit”—so your team can see where to focus revisions.
Letter of the law
How well does your labeling comply with the requirements and recommendations from key standards and guidance?
Spirit of the law
How well does your labeling reflect the intent of user-centered principles and risk reduction?
Why it matters
We’ve built this tool for clarity and speed. You’ll get targeted recommendations for improvement, rooted in standards, ready for action.

As many as 70% of
HF professionals cite poor IFU design as a top reason FDA reviewers request more data.

Labeling affects everything—use errors, training success, device comprehension, and submission timelines.

The IFU Scorecard is built on 600+ requirements across FDA CDER/CBER/CDRH, ANSI/AAMI, ISO/IEC/IEEE, EU MDR and more.
What we deliver
Composite score report (letter and spirit of the law)
Highlighted strengths and weaknesses
Specific recommendations for content and styling updates
Visual examples, where applicable
Optional re-score after revisions to show progress
Common challenges we solve
Whether you’re a small team without in-house expertise or a large organization navigating complex stakeholder input, we help you:
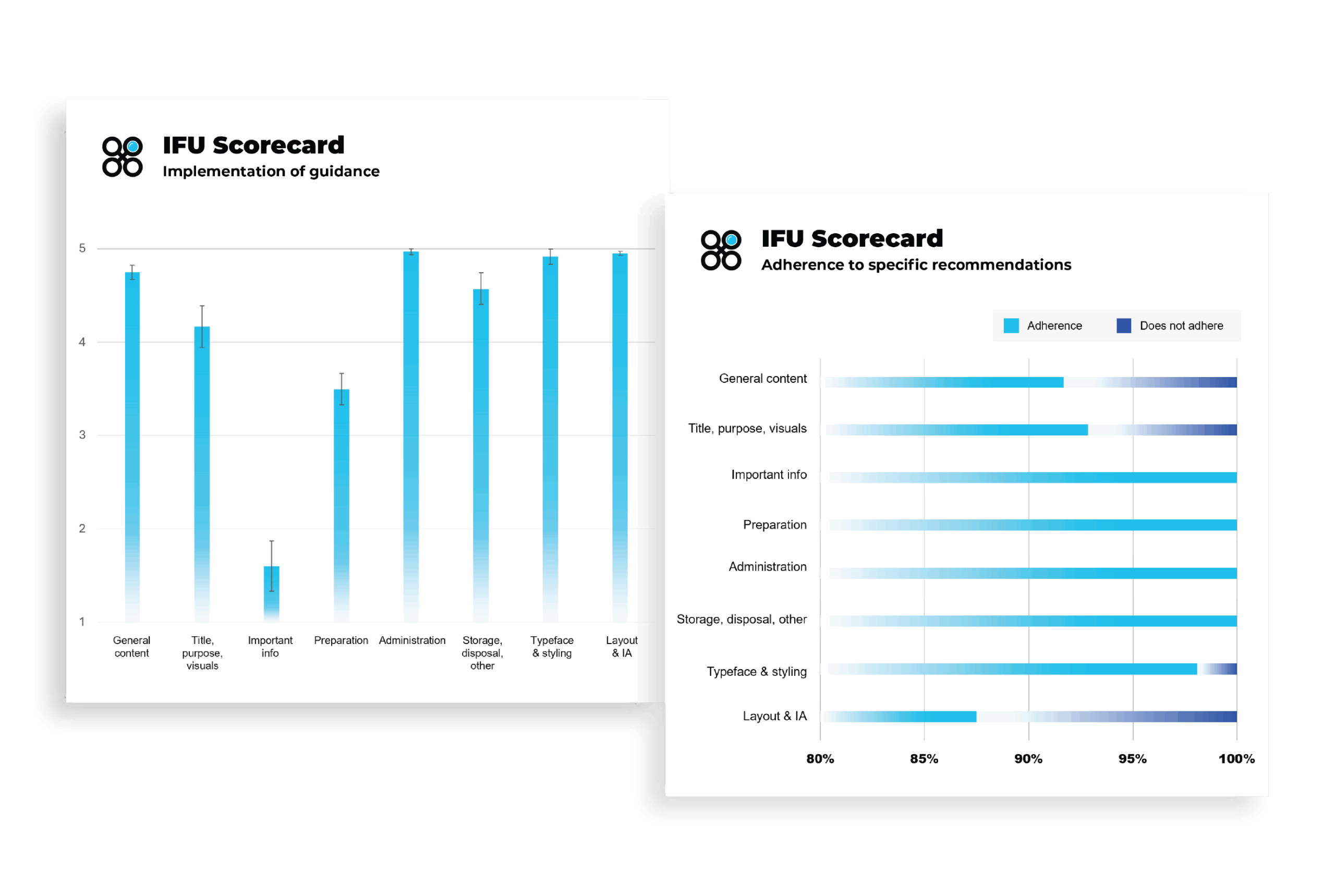
IFU Scorecard sample findings
Top strengths
Top opportunities
Your IFU is more than a document; it’s a device experience
In regulated environments, instructional design is not optional. It’s core to safety and success.
Let’s ensure your IFU and labeling are clear, compliant, and easy to use for regulators, providers, and patients.