Medical device research
We primarily work in healthcare, where we have experience across hundreds of products, in diverse use environments. We have a highly specialized medical device and med tech research practice — exceeding most consultancies in experience and scalability.
We partner with you to ensure safe and effective use at any stage

Experience matters
Our team’s experience is both broad and deep. Our clients benefit from the fact that 70% of our work over the past 5 years has been in the medical device and med tech space. Clients also benefit from the other 30% of work in other sectors. Our cross-training in research conducted in both the medical device and consumer technology sectors makes for well-rounded researchers equally adept in conducting free-flowing generative research, as well as more structured validation research.


%
of our work comes from repeat clients
%
of our work is early-stage research
FDA or CE mark (EU) submissions supported each year
Pharmaceutical companies have trusted us with their research
What do we test?
We gather data to refine and enhance the user experience while ensuring the safe and effective use of your product. We engage in a systematic and continuous process of usability testing, user feedback collection, and expert data analysis. This approach allows you to identify and address issues and opportunities for improvement before seeking validation.

Connected drug delivery & injection devices
We have over 15 years of experience conducting research with a wide range of injection and drug delivery devices. Deep relationships with recruitment vendors and clinical sites ensure access to a wide variety of patient populations and specialized healthcare professionals.
Clinical and home use
In clinical settings, delivery device design must account for variable workflows. Often for home use, we need to account for untrained or even distracted use cases. Our teams conduct on-site sessions, where we have a flexible lab approach as every home, office, and clinic is different.
Form-factor design
Form factor of injection devices can be critical for safe and effective use, particularly when dexterity and/or hand dysfunction are of concern. Our experts have years of experience and education in human factors research, including the cognitive usability and physical ergonomics aspects of human-product interaction.
Medication delivery and training
Clarity created around injection hold times, dosage calculations, and any necessary reconstitution procedures is essential.
Instructions for use (IFU)
The IFU plays an important role and should be developed along with the delivery device. Our expert IFU designers have worked within this regulated industry and have deep knowledge of FDA and CE guidance regarding this important component of the injection device.
Regulatory guidance
Beyond general guidance on application of HFE, we frequently communicate with the FDA, providing the most updated knowledge to construct arguments and mitigate FDA questions and concerns.
Test environment: encourage natural-use behavior
Each user group operates a device in various settings, requiring different environments to put them in their natural state while testing simulated drug delivery. Our lab capabilities adapt to the project’s needs, whether it’s observing simulated injections in a lab or real injections at a patient’s home, our team anticipates the challenges that might be presented in these varied settings.
Realistic injection locations
Manikins are used as patients or injection pads can be placed on the thighs, arms, or abdomen based on instructional materials.
Injection site
Participants select their preferred injection site prior to placing an injection pad.
Supplies match actual use
Gloves, alcohol swabs, hand sanitizer, cotton balls, trash cans, sharps container, etc. are provided.
Provide context, including prescribing scenarios
The understanding of realistic scenarios is deepened, e.g., HCPs are asked to imagine a patient is in the waiting room or to prepare an injection as they would in their office or simulating a prescribing scenario
Refrigeration
Cold storage affects viscosity and handling and is incorporated into protocols.

SaMD & digital health
In 1999, our team began in the software and website usability space. It’s in our DNA! Our deep expertise in this medium offers insight from every industry, across hundreds of user groups. Our clients benefit from this expertise and our strong consumer tech business that helps drive the latest innovations in software and app development. As your team works to build software as a medical device (SaMD), we support from both the software UX aspect as well as helping you to successfully navigate regulatory requirements of medical devices.
Supporting your SaMD development process
We have been supporting medical device development for many years! In fact, we helped author industry standards that are the basis for FDA human factors guidance. We help our clients to incorporate user feedback early and often in the development process as well as:
- Navigate FDA guidance documents.
- Determine the best research approach considering all constraints.
- Design SaMD with insights backed by user research.
- Identify areas where risk can be mitigated.
Get the user experience right and win the market
The lack of user-friendly solutions has been cited as a large barrier for prescribing digital therapeutics. We help you develop useful, usable, and engaging products through early-stage research to give patients and care providers the best-in-class experience that will catapult SaMD into the mainstream.

Packaging & IFUs
Our expertise and tools help you ease into and navigate the 160+ standards and regulations for packaging and IFUs.
Start down the road to FDA approval with an advantage
Save time and money
Receive feedback early and often to course correct while it’s still easy and more cost-effective to do so.
Ensure instructional materials comply
Be prepared earlier in the process for device regulatory and safety reviews.
Align design across device materials
Identify gaps and potential areas to better align your materials with cautions, guidelines, symbols, and warnings.
Support safe and effective use
Determine whether a packaging insert is going to facilitate the safe use of the device, ensuring it is not a hindrance, but communicates what it needs to.
Bold deliverables that inform your next step
We specialize in providing cohesive, consistent design, from wireframes to high-fidelity deliverables, that are built-out according to your specs and requirements, while adhering to regulatory guidelines.

Surgical tools, diagnostics, and robotics
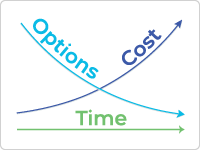
Involving users very early on in the design of surgical tools, diagnostic equipment, and robotics is especially important, where the costs of making late-stage changes are very high, or often not possible. To support this, we can conduct usability evaluations using non-functional prototypes to see beyond only assessing whether users successfully completed a task.
3D prototypes
We are versed in working with client provided 3-D printed prototypes, often used as shaped-like, feels-like models, representative of the full development of a product.
GUI prototypes
We specialize in creating graphical user interface (GUI) prototypes to use as interactive, clickable simulations of how a system’s display will look and function.
Ensure testing is representative
Gain a deep understanding of users
Our research helps to determine what users are trying to accomplish and uncover pain points currently experienced using similar devices.
Focus on micro-interactions
We iterate on every interaction, starting with the smallest, then begin to add additional layers, like complexity and parts of the workflow.
Consider user group experience levels
From recruiting individuals to recruiting full healthcare teams, we recommend the most representative approach that will organically mimic diverse experience levels.
Select the right training method
We work closely with you to determine, together, which method of training to use and whether to incorporate decay training into the process.
Remain flexible
We understand that plans evolve, and are adaptable to your needs, project preferences, and study dates. Our ISO 9001-certified processes and training offers the confidence that no matter which Bold Insight team members you engage with, you receive the best of the best quality and experience.
What do we deliver?
We offer a comprehensive set of deliverables to equip your project teams with the actionable data they need to make informed decisions and create exceptional user experiences. All deliverables are customized to your goals and needs and include our analysis and design recommendations where appropriate.
We help you navigate regulatory guidance to support a successful submission
We are in a unique position to leverage learnings across various submissions/interactions with the FDA (without compromising confidentiality). We help manufacturers navigate FDA guidance documents related to the application of human factors engineering to the development of medical and drug-delivery devices, help respond to direct feedback from FDA (e.g., support a response/action/change to FDA feedback on a protocol or report), and determine the best research approach taking all constraints into consideration.
For medical device manufacturers, industry standards and regulatory guidance demand validation of safe and effective use through application of human factors engineering and usability engineering (HFE/UE). We have been applying HFE/UE to medical device development longer than the FDA has been regulating it! Our research is conducted according to the guidelines found in IEC 62366-1, TR 62366-2, FDA Guidance on applying HFE/UE to medical device development, AAMI HE75, and other standards wherever appropriate.
ANSI/AAMI/IEC 62366-1; TR 62366-2
62366-1 specifies a process for a manufacturer to analyze, specify, develop and evaluate the usability of a medical device as it relates to safety. 62366-2 provides guidance for those implementing the process described in 62366-1.
FDA Guidance on applying HFE/UE
FDA guidance on applying human factors and usability engineering to medical devices intended to maximize the likelihood that new medical devices will be safe and effective for the intended users, uses and use environments.
ANSI/AAMI HE75
Human factors engineering – Design of medical devices. Covers general HFE principles, specific HFE principles geared towards certain UI attributes, and special applications of HFE (e.g., software UI, mobile medical devices, hand tools).
Recruiting specialized populations
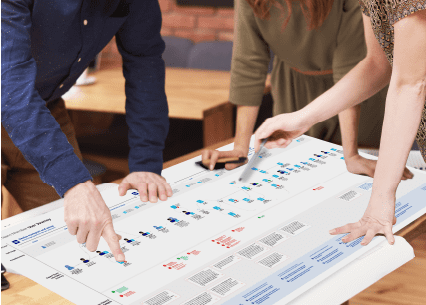
Our team has extensive experience interviewing and observing patient populations, healthcare professionals, and others who support patient care and disease management. We have managed HF studies ranging in size and complexity, up to samples of more than 250 participants, representing thousands of face-to-face hours, both in lab & home environments.

HCP populations with which we have conducted research:
Anesthesiologists, Cardiologists, Caregivers, Critical Care Doctors, Medical Assistants, Neurologists, Nurses, Nutritionists, Ophthalmologists, Opticians, Pharmacists, Primary Care Physicians, Psychiatrists, Social Workers, Surgeons, Technicians
Patient populations with which we have conducted research:
Alzheimer’s, Amblyopia, Ankylosing Spondylitis, Bipolar Disorder, Cancer, Crohn’s, Diabetes, Hemophilia, Major Depressive Disorder, Meibomian Gland Dysfunction, Migraine, Parkinson’s, Psoriasis, Rheumatoid Arthritis, Schizophrenia
Clinical sites and private practice recruiting support
If clinical sites or private practices are needed to support the recruit, prior to fieldwork, the clinical trial recruiter or private practice partner screens and schedules participants and backup participants through existing database and outreach. During fieldwork, they coordinate transportation of participant (and caregiver) to/from central test facility, if needed. They can also provide onsite HCP to conduct participant assessment prior to session and post-session assessment prior to dismissal. After fieldwork, our partners update the database with participation information and sources participants for future studies based on need.
Related content
Read our latest news & insights
Case studies
5 tips for designing AI-enabled SaMD that HCPs trust
Design an AI-enabled software as a medical device that offers cutting-edge capabilities and earns the trust of healthcare professionals, ultimately improving patient care.
Human factors testing for OTC drugs and non-prescription medical devices
As the field of human factors continues to evolve, so must our approaches to testing and study design. While the FDA’s 2013 guidance laid the groundwork for study design and methodology, recent discussions and conference presentations have prompted a shift in how we approach OTC HF testing, leading to more comprehensive and realistic assessments of these products.
Case studies