2025 HFES Health Care Symposium
Welcome, HFES 2025 attendees! We’re delighted to connect with you in Toronto and share insights on critical healthcare challenges, from cybersecurity and design validation to injection force and navigating sensitive health topics in HF studies.
The history of human factors can tell us a lot about our industry’s future. We brought this history to life with an interactive booth experience, taking you back in time through the milestones that give us a glimpse of where it might be headed.
Explore our services and how we support you! Together, we’re the future of HF!
We empower healthcare innovation
We primarily work in healthcare, where we have experience across hundreds of products, in diverse environments. We help you to identify and address issues and opportunities for improvement before seeking validation, ultimately leading to successful regulatory submissions. Learn more about our medical device research capabilities ›
What we test
- Companion applications
- Training programs
- Packaging and labeling
- SaMD & digital health
- Connected drug delivery
& injection devices
- Digital therapeutics
- Instructions for use
- Surgical tools
- Diagnostics
- Patient monitoring hardware
& software
How we do it
Paper research
- HF plan
- Task analysis
- Heuristics
- IFU Scorecard
- Use-related risk analysis
People research
- User needs
- Ethnography
- Iterative design
- Formative usability
- HF validation testing
Design AI-enabled SaMD that HCPs trust
Leverage decades of human factors and UX knowledge to build better AI tech. Healthcare has always demanded precision, empathy, and innovation. Now, as AI transforms our capabilities, the stakes have never been higher. We ensure your AI-driven solutions meet the rigorous demands of healthcare by aligning technology with user needs and regulatory standards.
Our decades-long global partnership
Bold Insight is a part of ReSight Global, a family of UX and human factors research agencies with offices in nine countries and coverage in over 100 key markets. Our broad set of tools and methods extends your reach and helps you understand users across borders, minimizing the challenges often associated with global user research.
Navigating human factors in China?
For all our China-based research, we work closely with our sister company, XplusX, based in Shanghai. XplusX specializes in the healthcare industry and has closely followed the evolution of regulatory guidance. With deep market knowledge and hands-on expertise in device testing, we support clients through every stage of product development and launch in China.
Download our HFES poster presentations
Integrating FDA’s new cybersecurity guidance into medical device human factors engineering processes
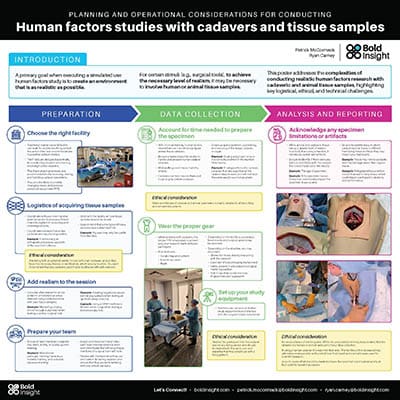
Planning and operational considerations for conducting human factors studies with cadavers and tissue samples
Warm up to clarity: Iconography in temperature communication on combination products and medical devices
Navigating sensitive health topics and tasks in human factors testing: Strategies for research validity and participant well-being
“This would never happen in my hospital” – The importance of adjusting your simulated use environment based on participant feedback
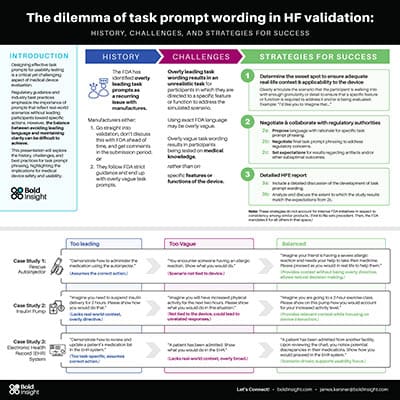
The dilemma of task prompt wording in HF validation: History, challenges, and strategies for success
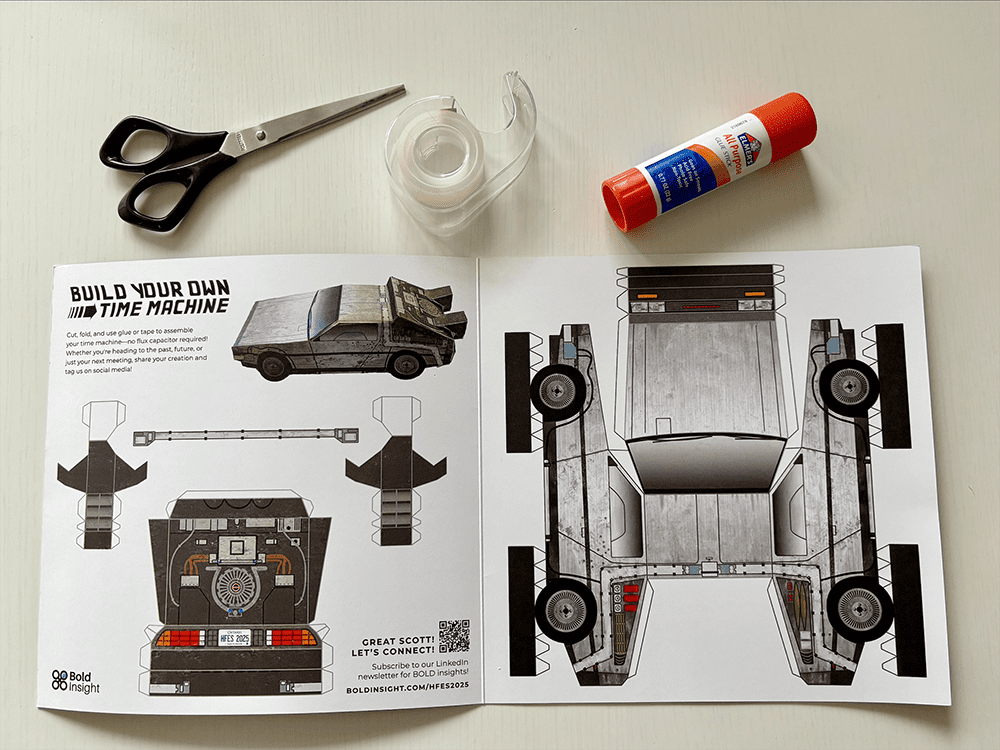
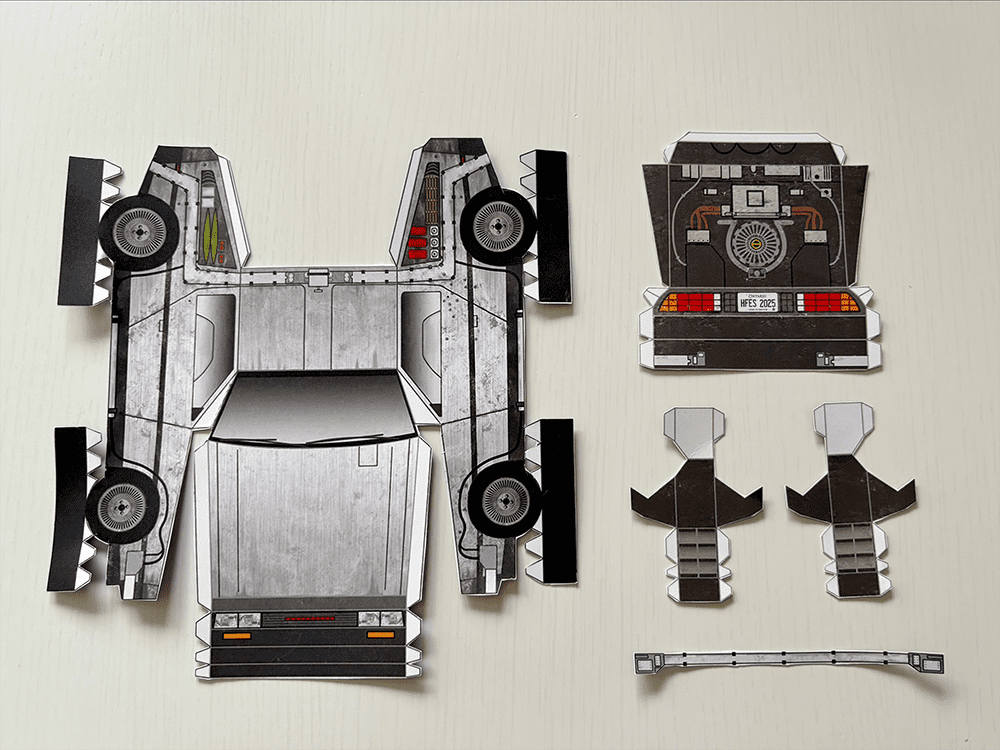
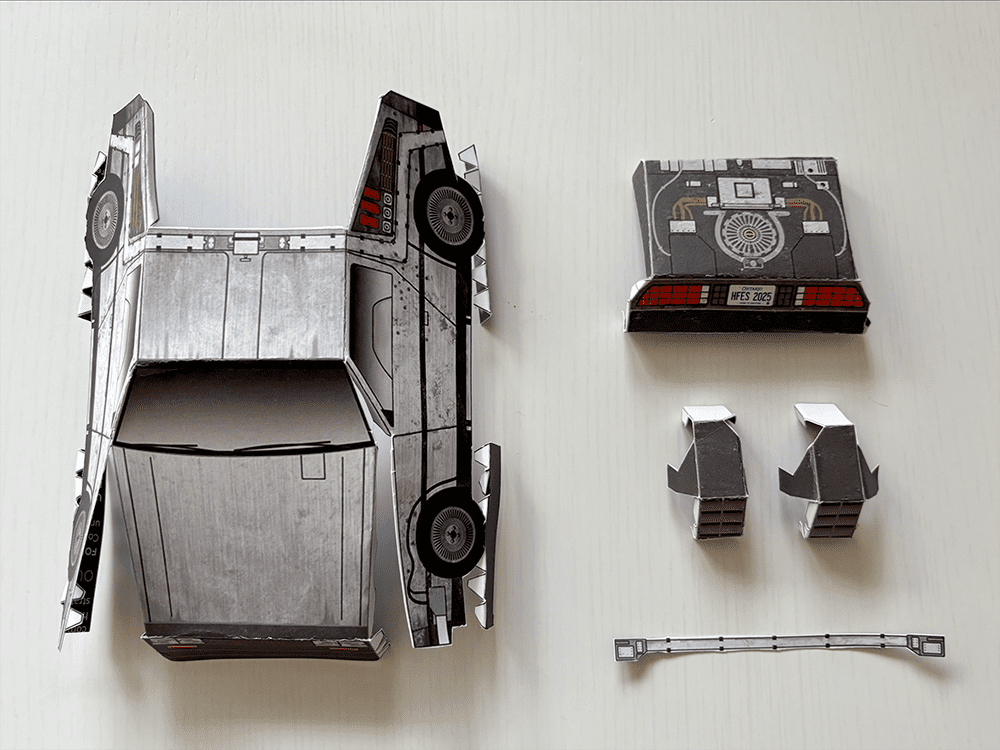
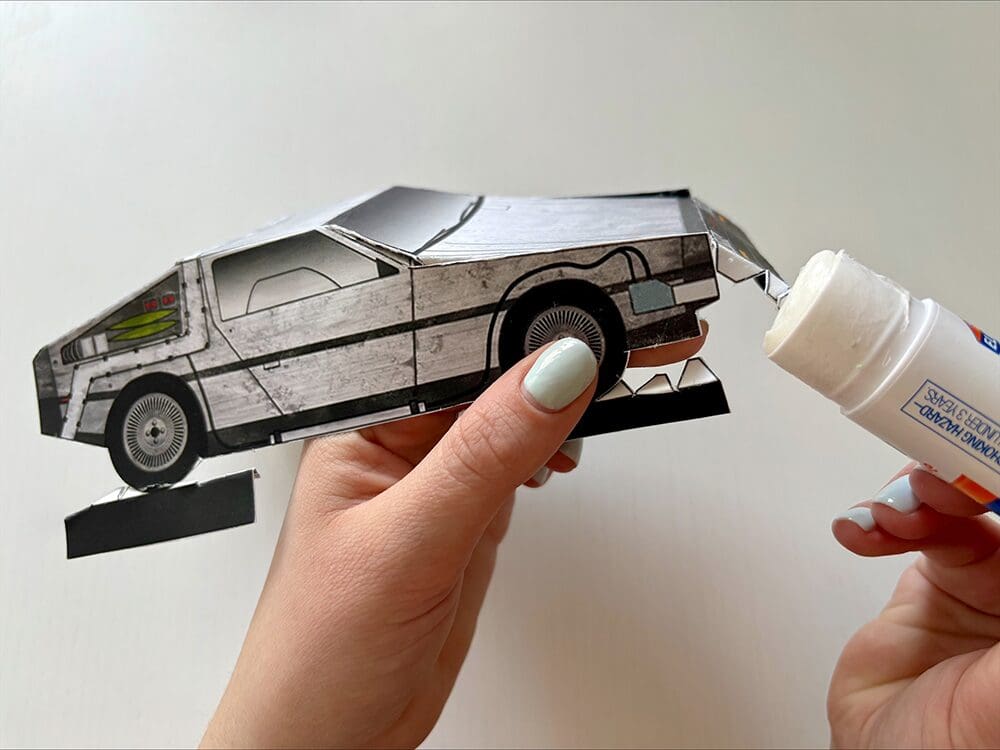
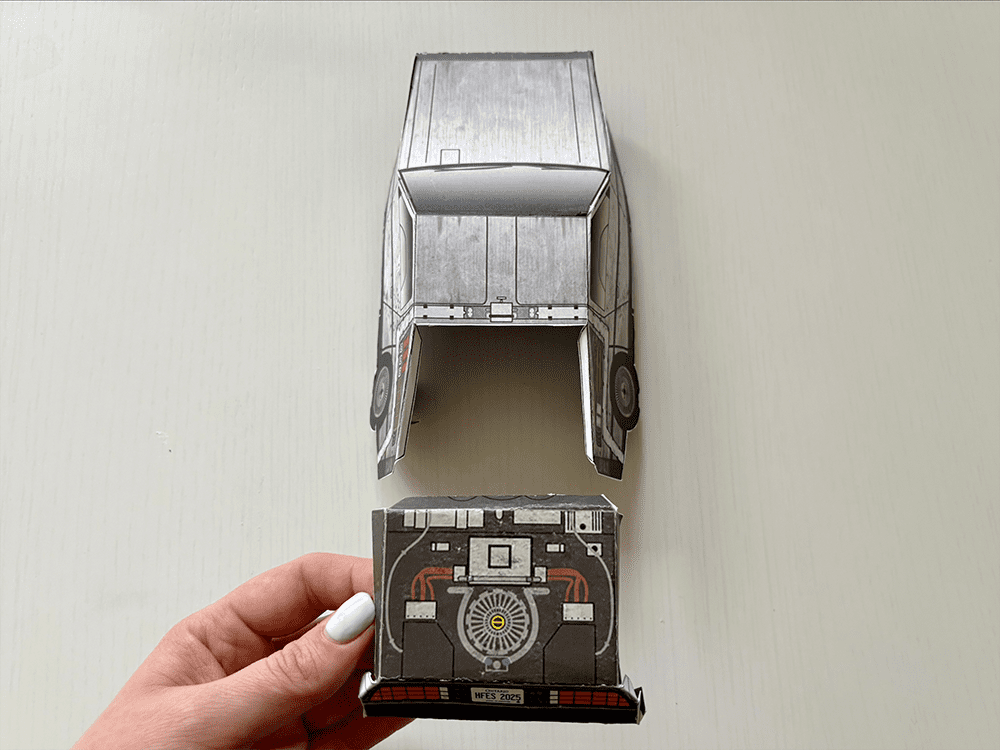
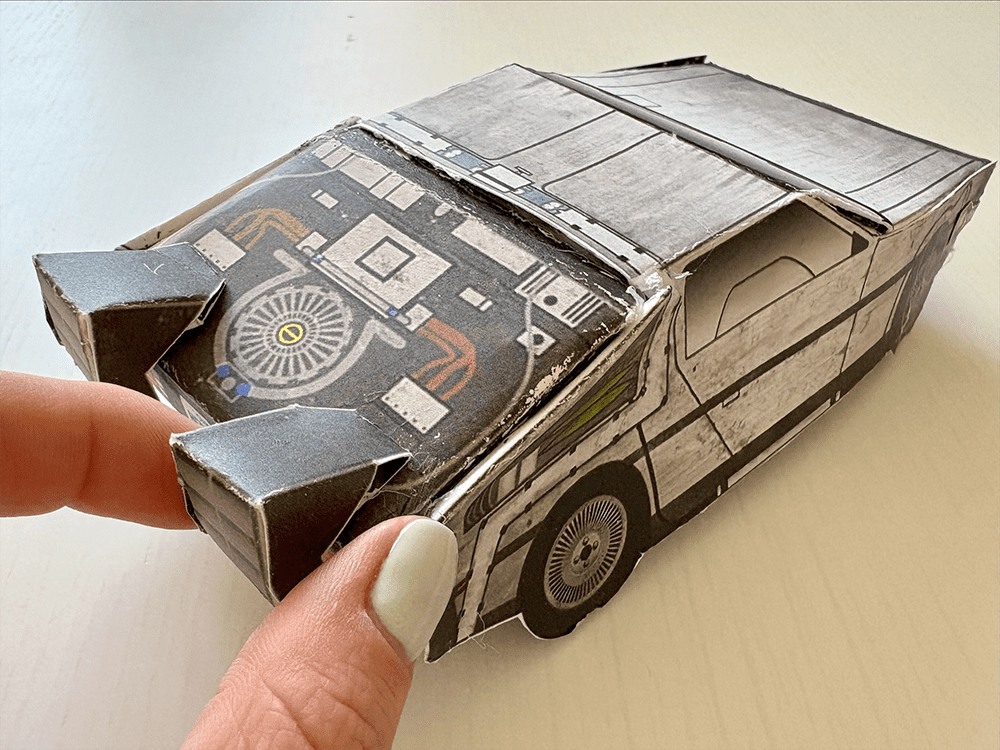
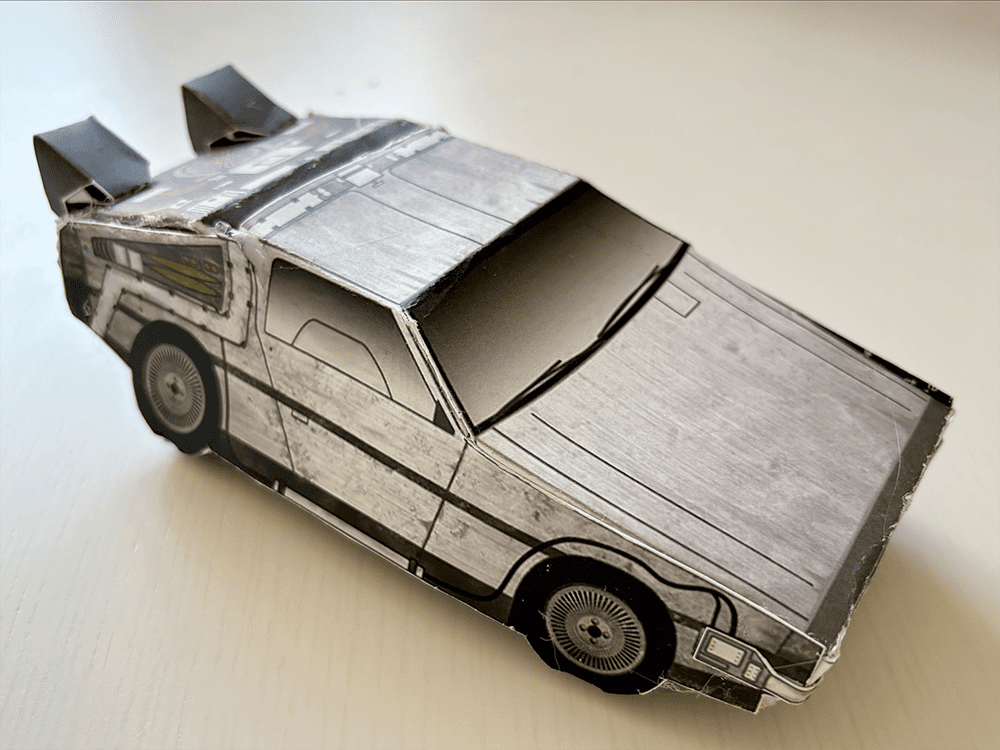
Tutorial: Build your own time machine
1.21 gigawatts of fun await! Inside the Bold Insight insert from your conference bag, you’ll find a cutout DeLorean. Follow our step-by-step tutorial and before you know it, you’ll be ready to reach 88 MPH!
Once you’ve assembled your nostalgic creation, snap a photo and share it on social media. Tag @boldinsight so we can celebrate your journey through time!
Jam out to the Bold Beats album
Enjoy our AI-generated music and lyrics about UX and HF research! We’ve guided the evolution of artificial intelligence, even writing one of the first books on AI and UX. So, when we get our hands on the latest AI tools, we can’t resist diving in. The result? A collection of songs that are sure to make you laugh (and maybe even dance!)
Available on Spotify, Apple Music, Amazon Music, and YouTube Music.
LIMITED EDITION!
Ergo-nna Go to Toronto (HFES edition) exclusive single
Photo booth stream
HFES attendees stepped into the future of HF at our photo booth. Now, you can relive the fun! Check out our gallery of pictures taken throughout the conference.
Subscribe to our monthly newsletter
Design smarter, innovate faster—user-centered strategies that win in the market.
Get exclusive insights and strategies with our newsletter, where we share real client challenges and the bold approach that led to their success. Enhance your competitive edge and maximize ROI with each issue.